Abstracts
The aim of this cross sectional study was to evaluate bone mineral density (BMD) and serum levels of 25-hydroxy vitamin D (25OHD) in a group of patients taking antiepileptic drugs (AED) for a seizure disorder. Between May-2001 and January-2003, we evaluated 58 patients (40 women/18 men), 34.4±6 years old living in Curitiba or in its metropolitan area, on antiepileptic therapy for 2 to 38 years (10 on monotherapy /48 on multiple drugs regime). The group was matched by age, gender, and bone mass index to 29 healthy subjects (20 women/ 9 men); 34.2±5.9 years old. Medical history and physical exam were performed on all subjects with particular information sought about fractures and risks factors for osteoporosis. Blood samples were collected for total serum calcium, albumin, phosphorus, creatinine, total alkaline phosphatase, and liver function tests. BMD of the lumbar spine, femur and forearm was determined by dual energy X-ray absorptiometry (DXA, Hologic QDR 1000). Between February and April-2003, other blood samples were collected to measure 25OHD, intact paratohormone (PTH) and calcium. Unemployment and smoking history were more frequent among patients than among controls (p<0.05). Fifteen patients had a fracture history, all of which occurred during a seizure. The BMD of the lumbar spine (0.975±0. 13 g/cm² vs. 1.058±0.1 g/cm²; p<0.03) and of the total femur (0.930±0.1 g/cm² vs. 0.988±0.12 g/cm²; p<0.02) was lower in patients than in controls. In 63.5% of patients and in 24.1 % of controls a T-score < -1.0 in at least one site was seen. The AED users had higher total alkaline phosphatase and lower 25OHD (p<0.02). No correlations between BMD and 25OHD were found. The use of phenytoin was correlated with a greater incidence of fractures (RR: 2.38). We conclude that patients on chronic use of AED have alterations in bone metabolism characterized in this study by lower BMD of the lumbar spine and total femur and lower serum concentrations of 25OHD.
Enzyme-inducing antiepileptic drugs; 25OH vitamin D; osteomalacia
O objetivo deste estudo transversal foi avaliar a densidade mineral óssea (DMO) e os níveis de 25hidroxi vitamina D (25OHD) em um grupo de pacientes com epilepsia e usuários crônicos de drogas antiepilépticas (DAE). Entre maio-2001 e janeiro-2003 avaliamos 58 pacientes (40 mulheres/18 homens) residentes em Curitiba ou região metropolitana da cidade, com média de idade 34,4±6 anos e tempo de tratamento entre 2 e 38 anos (10 em monoterapia/48 em politerapia). O grupo de pacientes foi emparelhado por idade, sexo e índice de massa corpórea com 29 indivíduos aparentemente sadios (20 mulheres/9 homens; 34,2±5,9 anos). Pacientes e controles foram submetidos a anamnese e exame clínico, com ênfase na história de fraturas e fatores de risco para osteoporose. Nas visitas foram coletadas amostras de sangue para dosagens de cálcio, albumina, fósforo, creatinina, fosfatase alcalina, transaminases e gama GT. Foi avaliada também a DMO na coluna lombar, fêmur e antebraço (DEXA, Hologic QDRW1000®). Entre fevereiro e abril-2003, pacientes e controles foram chamados para nova coleta de sangue para dosagem da 25OHD e parato-hormônio (PTH) intact. Desemprego e tabagismo foram mais comuns nos pacientes do que nos controles (p<0,05). Quinze pacientes relataram fraturas durante as crises epilépticas. A DMO da coluna lombar (0,975±0,13 g/cm² vs 1,058±0,1 g/cm²; p<0,03) e do fêmur total (0,930±0,1 g/cm² vs 0,988±0,12 g/cm²; p<0,02) foi menor nos pacientes do que controles. Em 63,5% dos pacientes e em 24,1% dos controles foi registrado escore T < -1.0 desvio-padrão em pelo menos um dos sítios avaliados. Os usuários crônicos de DAE apresentaram níveis de fosfatase alcalina mais elevados (p<0,01) e níveis de 25OHD mais baixos (p<0,02 vs controles). Não houve correlação entre a DMO e os níveis de 25OHD. O uso de fenitoína correlacionou-se positivamente com maior incidência de fraturas (RR: 2,38). Concluímos que usuários crônicos de DAE apresentam importantes alterações do metabolismo mineral ósseo, demonstrada no presente estudo através de valores menores da DMO em coluna lombar e fêmur e níveis séricos diminuídos de 25OHD.
drogas antiepiléticas indutoras hepáticas; 25OH vitamina D; osteomalácea
Densidade mineral óssea e níveis séricos de 25 OH vitamina D em usuários crônicos de drogas antiepilépticas
Carolina A.M. KulakI; Victória Z.C. BorbaI; John P. BilezikianII; Carlos E. SilvadoIII; Luciano de PaolaIII; César L. BoguszewskiI
IServiço de Endocrinologia e Metabologia do Hospital de Clínicas da Universidade Federal do Paraná (UFPR) Curitiba PR, Brasil
IIDepartments of Medicine and Pharmacology, College of Physicians & Surgeons, Columbia University, New York, NY, USA
IIIServiço de Neurologia do Hospital de Clínicas da UFPR
CorrespondenceCorrespondence to Dra. Carolina Aguiar Moreira Kulak Rua Padre Camargo 262 80060-240 Curitiba PR - Brazil E-mail: kulakjc@uol.com.br
ABSTRACT
The aim of this cross sectional study was to evaluate bone mineral density (BMD) and serum levels of 25-hydroxy vitamin D (25OHD) in a group of patients taking antiepileptic drugs (AED) for a seizure disorder. Between May-2001 and January-2003, we evaluated 58 patients (40 women/18 men), 34.4±6 years old living in Curitiba or in its metropolitan area, on antiepileptic therapy for 2 to 38 years (10 on monotherapy /48 on multiple drugs regime). The group was matched by age, gender, and bone mass index to 29 healthy subjects (20 women/ 9 men); 34.2±5.9 years old. Medical history and physical exam were performed on all subjects with particular information sought about fractures and risks factors for osteoporosis. Blood samples were collected for total serum calcium, albumin, phosphorus, creatinine, total alkaline phosphatase, and liver function tests. BMD of the lumbar spine, femur and forearm was determined by dual energy X-ray absorptiometry (DXA, Hologic QDR 1000). Between February and April-2003, other blood samples were collected to measure 25OHD, intact paratohormone (PTH) and calcium. Unemployment and smoking history were more frequent among patients than among controls (p<0.05). Fifteen patients had a fracture history, all of which occurred during a seizure. The BMD of the lumbar spine (0.975±0. 13 g/cm2vs. 1.058±0.1 g/cm2; p<0.03) and of the total femur (0.930±0.1 g/cm2vs. 0.988±0.12 g/cm2; p<0.02) was lower in patients than in controls. In 63.5% of patients and in 24.1 % of controls a T-score < -1.0 in at least one site was seen. The AED users had higher total alkaline phosphatase and lower 25OHD (p<0.02). No correlations between BMD and 25OHD were found. The use of phenytoin was correlated with a greater incidence of fractures (RR: 2.38). We conclude that patients on chronic use of AED have alterations in bone metabolism characterized in this study by lower BMD of the lumbar spine and total femur and lower serum concentrations of 25OHD.
Key words: Enzyme-inducing antiepileptic drugs, 25OH vitamin D, osteomalacia.
RESUMO
O objetivo deste estudo transversal foi avaliar a densidade mineral óssea (DMO) e os níveis de 25hidroxi vitamina D (25OHD) em um grupo de pacientes com epilepsia e usuários crônicos de drogas antiepilépticas (DAE). Entre maio-2001 e janeiro-2003 avaliamos 58 pacientes (40 mulheres/18 homens) residentes em Curitiba ou região metropolitana da cidade, com média de idade 34,4±6 anos e tempo de tratamento entre 2 e 38 anos (10 em monoterapia/48 em politerapia). O grupo de pacientes foi emparelhado por idade, sexo e índice de massa corpórea com 29 indivíduos aparentemente sadios (20 mulheres/9 homens; 34,2±5,9 anos). Pacientes e controles foram submetidos a anamnese e exame clínico, com ênfase na história de fraturas e fatores de risco para osteoporose. Nas visitas foram coletadas amostras de sangue para dosagens de cálcio, albumina, fósforo, creatinina, fosfatase alcalina, transaminases e gama GT. Foi avaliada também a DMO na coluna lombar, fêmur e antebraço (DEXA, Hologic QDRW1000®). Entre fevereiro e abril-2003, pacientes e controles foram chamados para nova coleta de sangue para dosagem da 25OHD e parato-hormônio (PTH) intact. Desemprego e tabagismo foram mais comuns nos pacientes do que nos controles (p<0,05). Quinze pacientes relataram fraturas durante as crises epilépticas. A DMO da coluna lombar (0,975±0,13 g/cm2vs 1,058±0,1 g/cm2; p<0,03) e do fêmur total (0,930±0,1 g/cm2vs 0,988±0,12 g/cm2; p<0,02) foi menor nos pacientes do que controles. Em 63,5% dos pacientes e em 24,1% dos controles foi registrado escore T < -1.0 desvio-padrão em pelo menos um dos sítios avaliados. Os usuários crônicos de DAE apresentaram níveis de fosfatase alcalina mais elevados (p<0,01) e níveis de 25OHD mais baixos (p<0,02 vs controles). Não houve correlação entre a DMO e os níveis de 25OHD. O uso de fenitoína correlacionou-se positivamente com maior incidência de fraturas (RR: 2,38). Concluímos que usuários crônicos de DAE apresentam importantes alterações do metabolismo mineral ósseo, demonstrada no presente estudo através de valores menores da DMO em coluna lombar e fêmur e níveis séricos diminuídos de 25OHD.
Palavras-chave: drogas antiepiléticas indutoras hepáticas, 25OH vitamina D, osteomalácea.
Epilepsy is a chronic condition characterized by recurrent clinical events or epileptic crises, which occur in the absence of a metabolic- toxic disease or fever. World epidemiologic data place the incidence of this disease from 11 to 131/100000, individuals per year1. To prevent seizures, antiepileptic or anticonvulsant drugs (AED) are used. The AED in common use are carbamazepine (CBZ), phenytoin (DPH), phenobarbitone (PB), sodium valproate (VPA), primidone (PRM), lamotrigina (LTG) and the benzodiazepines, clobazan (CLB) and clonazepan (CZP). The chronic use of AED can affect bone and mineral metabolism2-6. Studies indicate that 20 to 60% of AED users can develop rickets or osteomalacia, especially among older and institutionalized patients. However, in outpatient populations, some studies have not demonstrated a deleterious effect of AED on bone metabolism7-9. Drugs such as CBZ, PB, PRM and DPH are known to induce hepatic mixed function oxidase activity throughout the microsomal enzymes (P450) in the liver. They are thought to affect bone and mineral metabolism indirectly by increasing the metabolism of vitamin D, thereby causing vitamin D insufficiency or deficiency2,3,6,10. The other mechanism of the AEDs involves a direct action on bone cells, increasing bone resorption and formation. In this way, the AED may influence bone turnover4,5,13. Both mechanisms can be associated with a reduction in bone mineral density (BMD)5,6,11,12. Furthermore, DPH interferes with cation transport in many tissues, directly inhibits intestinal calcium transport and together with PB has been shown to inhibit vitamin D-mediated calcium absorption. In contrast, other AEDs that are descriptively termed hepatic noninducers, do not affect vitamin D metabolism. Finally, VPA affects bone metabolism by mechanisms that have not yet been identified.
The most common laboratory abnormalities described in relation to AED are hypocalcaemia, hypophosphatemia, elevated levels of alkaline phosphatase (AP), reduced levels of 25-hydroxy vitamin D (25OHD) and increased serum parathyroid hormone (PTH)3,4,10. The chronic use of AED is a well-known cause of secondary osteoporosis14,15. However, the investigation of potential abnormalities of bone mineral metabolism has not generally been emphasized in AED users. A survey by Valmadrid et al.16, revealed that only 28 and 41% of pediatric and adults neurologists, respectively, routinely investigate bone metabolism in their patients who were taking AEDs. Moreover, less than 10% of physicians who participated in the survey prescribed prophylactic calcium and vitamin D to their patients on AEDs. Since seizure disorders are chronic and persist throughout life, it is very important to investigate and prevent metabolic bone disturbances in these individuals, particularly since many have other risk factors for osteoporosis such as lack of physical activity and reduced exposure to sunlight3. Moreover, increased fractures rates have been described in patients with epilepsy17,18,19.
The aim of this study was to evaluate serum levels of 25OHD and bone mineral density in a group of ambulatory patients on AED, living in the city or environs of Curitiba, located in South of Brazil (25º South and 49º West).
METHOD
Study design This comparative, cross-sectional study evaluated 58 patients with epilepsy followed in the Outpatient Epilepsy Clinic of the Hospital de Clínicas da Universidade Federal do Paraná in Curitiba, between May 2001 and January 2003. The patients were compared to 29 healthy subjects, matched by age, gender and body mass index (BMI), recruited by advertisement and posters. This protocol was approved by the local Institutional Ethic Committee in Research of Human, and informed consent was obtained from all participants.
Inclusion criteria were women and men living in Curitiba or its environs; age equal to or greater than 25 years; use of AED for at least 1 year; regular menses (for women); and willingness to participate in the study. Inclusion criteria for the control group were individuals living in Curitiba or its environs, not taking AED, and willing to participate in the study. Women had to have regular menses. Each control subject was gender, age and BMI matched to two patients. Exclusion criteria for both groups were mental retardation; immobilization; overt bone deformities and the presence of conditions known to affect bone metabolism such as hepatic, hematological, rheumatologic or renal disorders, hyperparathyroidism, osteogenesis imperfecta, hyperthyroidism, gastrointestinal disorders (e.g., malabsorption), hypogonadism, medications known to affect bone turnover e.g. glucorticoids, bisphosphonates, thiazides, anticoagulants, GnRH analogues, steroids.
All patients provided information about time of diagnosis and treatment of epilepsy, frequency of seizures, type and dose of AED and previous fractures. A questionnaire assessing the risk factors of osteoporosis such as smoking, alcohol use, calcium and caffeine intake, lack of physical activity, family history of osteoporosis was recorded for all subjects. A complete medical history and physical exam was performed. BMI was calculated by weight in kilograms (kg) divided by the height in square meters (m2).
Average daily dietary calcium intake was estimated from reported intake of dairy products, and categorized into three tertiles: < 400 mg, 400-800 mg, and >800mg. Because caffeine content of brewed beverages varies widely, the caffeine content of 8 ounces of coffee, tea, and cola drinks was set at 100 mg, 47 mg, and 40 mg, respectively (20). Average daily caffeine intake was categorized as low (<200 mg), medium 200-400 mg and high (>400 mg). Physical activity was considered moderate when it was performed on a regular basis at least three hours per week.
On the same day of the interview, blood samples were collected for measurement of albumin, phosphorus, creatinine, AP, and liver function tests. At the same visit, BMD measurements of the lumbar spine, femur and forearm (DXA, Hologic QDR 1000) were made. Between February and April-2003, more blood samples were obtained in order to measure 25OHD, total testosterone, intact PTH and calcium, to account for a possible seasonal variation in serum 25OHD levels.
Biochemical measurements Intact parathyroid hormone was measured in duplicate by immunochemiluminescent assay (DPC, Los Angeles, USA). The detection limit was 1 pg/ml. Intra-assay variability was less than 5.7% within the concentrations range of 72 - 662 pg/ml (normal range, 7 - 53 pg/ml). The 25OHD was measured in duplicate by radioimmunoassay (RIA; DiaSorin, Minnesota, USA). The detection limit was 5 ng/ml. The intra-assay variability was less than 12.5% within the concentrations range of 8.6 to 49 ng/ml (normal range, 9 to 37 ng/ml). In this protocol, vitamin D deficiency was defined by serum levels of 25OHD less than 10 ng/ml. Insufficiency was defined as a 25OHD between 10 and 20 ng/ml21. Total serum testosterone was measured in duplicate by electrochemiluminescent assay (Roche Diagnostics GmbH, Mannheim, Germany). The detection limit was 0.02 ng/ml (0.069nmol/l). Intra-assay variability was lower than 4.6% within the concentrations range 0.24 - 3.45 ng/ml (normal range, 280- 880 ng/ml).
Bone mineral density BMD was performed by dual-energy X-ray absorptiometry on a Hologic QDR - 1000 W (Hologic, Inc., Waltham, MA). All tests were performed by the same technician. The coefficient of variability was 0.46% at the lumbar spine and 0.52% at the proximal femur. BMD was expressed as g/cm2 and as T and Z scores. The World Health Organization (WHO) defines osteoporosis as a T- score < -2.5 and osteopenia as a T-score between -1.0 and -2.49. Subjects with T-scores >-1.0 are considered to have normal BMD. These criteria have been established only for Caucasian postmenopausal women, but we and other have applied these WHO criteria also to premenopausal women and men22. Sites analyzed were lumbar spine (L1 to L4), proximal femur (femoral neck and total hip) and forearm (ultra distal, total radius and distal 1/3 radius site).
Statistical analysis The patients and controls were matched 2:1, where one healthy subject presented with age, gender and BMI similar to two patients of the epilepsy group. This correlation, 2 cases by 1 control, was sufficient to maintain statistical power. The variables were analyzed initially by normality tests, covariates and histogram analyses. Distribution curves were expressed as means ± SD. Asymmetric distribution was expressed as median values. In the univariate analyses, for continuous variables, normal distribution used parametric tests: Student's t-test and ANOVA were used for repetitive measurements. For the variables that displayed asymmetric distribution, non-parametric tests were applied: Wilcoxon, Fisher and McNemar tests with calculation of the odds ratio. The X2 test was used in the evaluation of intake of calcium and caffeine, which were established as graded values. Correlations among two continuous variables were run by the Pearson test and in the multiplevariable analysis, models of logistic regression, multiple regression and discriminate analysis were used. Significance was set at p < 0.05.
RESULTS
Group epilepsy A total of 58 patients, 40 (69%) women and 18 men (31%), 25-47 years old (34.4 ± 6) were studied (Table 1). Thirty one (53.4%) patients were unemployed at the time of the study. The mean duration of treatment with AED varied from 2 to 38 years, with a median of 18.5 years. At the time of the study, 10 patients (17.2%) were on monotherapy and 48 (82.7%) were on multiple drug regimens. The most used AED was CBZ, followed by PB and DPH (Table 2). Thirty-seven patients (64%) presented with focal symptomatic epilepsy, of whom 2 (3.5%) were generalized idiopathic, and 17 (29%) were focal cryptogenic. Two patients (3.5%) were not identified as being partial or generalized23. Regarding the classification of seizures, fifty four patients (93%) had simple or complex partial seizure or partial seizure that are secondarily generalized, four (7%) had primarily generalized tonic-clonic seizures and two (3.5%) had a typically juvenile myoclonic epilepsy, presenting with myoclonic and tonic-clonic seizures24. With regard of control of crises, a median of 4 episodes a month (0-60) was related by the patients.
Control group Twenty nine healthy subjects (20 women and 9 men), mean age, 34.2 ± 5.9 years (25-45), matched to the epilepsy group were included in the study. In this group, all the participants were Caucasians and everyone was employed.
Risk factors to osteoporosis Among risk factors for osteoporosis, the only one that differed was smoking, with a greater incidence in the epilepsy group (Table 1).
Biochemical measurements The results of biochemical measurements of the study group are shown in Table 3. Mean results of routine biochemical testing (total calcium, albumin, phosphorus, magnesium, creatinine, AP, transaminases, and PTH were normal and did not differ statistically. In the male group, no statistical difference in serum levels of testosterone was found. However, 8 (44.4%) patients and 2 (6.8%) subjects from the control group had serum levels lower than 280 mg/dl. AP was higher in the epileptic group than the control (144.7 ± 49.3U/l vs 111.4 ± 32.7 U/l; p = 0.01), as was the case for gamma GT levels (46.5 ± 37.2 U/I vs 19.0 ± 10.6; p = 0.001). In contrast, AED users had lower levels of 25OHD (28.2 ± 10.3 ng/ml vs 34.4 ± 12.7 ng/ml; p = 0.02) (Fig 1). Twenty patients (34.4%) and two controls (6.8%) had levels of 25OHD below 20 ng/dl (10-20 ng/dl), indicating a greater incidence of vitamin D insufficiency in the AED users than in the control group (p=0.01). Although no subjects in the study had levels of vitamin D below the lower limit of the reference range (i.e., < 10 ng/ml), the physiological lower limit of normal (< 20 ng/ml) was found frequently in the study subjects. Lower levels of 25OHD correlated with absence of work (p= 0.04).
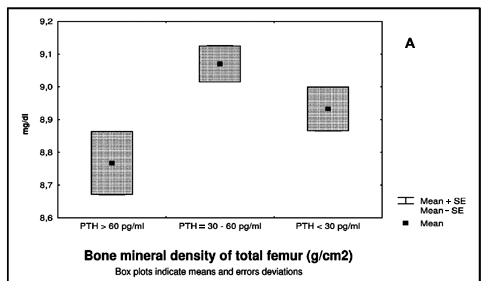
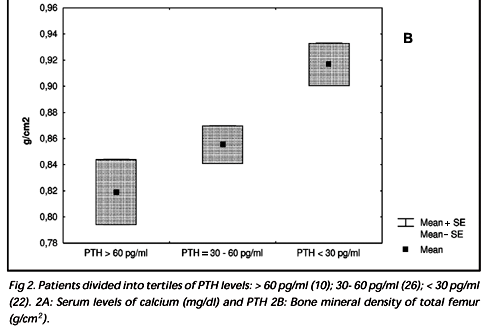
In the patient group, the mean serum level of PTH was 39pg/ml (18-88). No correlation was found between the PTH and BMD, serum levels of 25OHD, or calcium. However, when the patients were divided into tertiles of PTH [a. > 60 (n= 10), b. PTH between 30 and 60 (n=26), and c. PTH < 30 pg/ml (n=22)], it was observed that patients in the highest tertile had lower levels of calcium (p <0.03) (Fig 2a). Patients in this highest tertile of PTH showed BMD of the proximal femur that was in the lower tertile (p<0.02 femur neck, p< 0.03 total femur) (Fig 2b).
Bone mineral density There was no correlation between BMD at all sites measured and the time of treatment with AED. Similarly, no correlation was found between BMD and serum levels of 25OHD with the exception of a weak correlation at the femoral neck (RR: 0.53). The absolutes values of BMD are shown in Table 4. When WHO criteria were applied, osteopenia (T-score -1.0 to -2.49) of at least 1 site was diagnosed at 31 patients (53.4%) as compared to only 7 controls (24.1%). Osteoporosis by T-score (<-2.5) was observed to occur in 7 patients (10.3%), and no cases was diagnosed in the control group. Lumbar spine BMD was significant lower in patients than in control subjects (0.975 ± 0.13 g/cm2vs 1058 ± 0.1 g/cm2; p = 0.03). Twenty one patients (36.2%) and 2 controls (6.9%) had osteopenia at this site. Osteoporosis was diagnosed in 5 patients (8.6%). Similarly, total femur BMD of patients was significantly lower than BMD of the subjects from the control group (0.930 ± 0.1 g/cm2vs 0.988 ± 0.12 g/cm2; p = 0.02). Osteopenia of this site was found in 12 patients (17.2%) but in only 2 controls (6.9%), while osteoporosis was diagnosed in one patient. At the femoral neck, no difference between the groups was observed BMD of forearm was similar between both groups.
Types of AED and abnormalities of bone metabolism The PTH levels of patients who had been exposed to DPH and PB were significantly higher than the levels of PTH in patients who never had used these AEDs. The total femoral BMD was significant lower in those patients who had used PB (p= 0.03).
AED users and previous fractures Fifteen patients (25.8%) of the epilepsy group, five men and 10 women, with mean age of 36.3 years and mean BMI of 23.5 kg/m2, reported fractures related to a seizure episode. No association was observed between the presence of fractures and type of seizures, nor with risk factors for osteoporosis. DPH was the most frequent AED among these patients {relative risk=2.38 (1.31- 4.34)}. No significant difference was observed between the BMD of the patient with history of fracture than the patient without a fracture; however 11 (73.3%) with previous fractures presented T score < -1.0 at the lumbar spine.
The serum levels of 25OHD tended to be lower in the group who sustained fractures (p=0.08), with an incidence of 33% of vitamin D insufficiency in this group.
DISCUSSION
Our study has evaluated 58 young adults with epilepsy and on chronic AEDs. We have demonstrated significant abnormalities of bone metabolism, characterized by reduced BMD, reduced 25OHD, and increased AP. Previous studies have related these findings to chronic use of AEDs5,6,11,12. Serum levels of calcium and PTH were normal and similar to the control group. In fact, hypocalcaemia and secondary hyperparathyroidism, abnormalities initially described as part of a picture of anticonvulsant rickets or osteomalacia2,3,10, were not observed in our study. However, when we separated the patients into subgroups as a function of PTH levels, a profile of secondary hyperparathyroidism with lower BMD at the proximal femur emerged (Fig 2b). These data indicate that variable degrees of abnormalities in bone metabolism are present in users of AED. On the other hand, the majority of the patients with hypovitaminosis D presented with normal levels of PTH that may be explained, in part, by an elevated intake of calcium of these individuals or due to abnormal parathyroid gland responsiveness caused by the AED.
A relevant finding of this study was the significant reduction of BMD in the lumbar spine and total femur when compared to the control group. According to WHO criteria, 63, 5% of the patients had a diagnosis of osteopenia / osteoporosis in at least one of the sites measured. This incidence is higher than uncontrolled studies reported in the literature6,11,12. Because of the cross-sectional nature of the present study, it was not possible to know if the bone loss is specifically related to the use of AEDs or if there are other reasons for the differences appreciated in this study. Andress et al.11 evaluated the BMD of a group of AED users prospectively and demonstrated a rate of bone loss of 1.8% per year.
The greatest importance of a reduction BMD is as a predictor of fracture incidence, a point well documented in the literature25. Other studies, especially in institutionalized patients, have demonstrated increased fracture rates in patients with epilepsy17,18,19. In our study, 25.8% of the patients reported seizure-related fractures, which was not surprisingly as many of our patients had uncontrolled seizures and required multiple drugs regime with high doses of AEDs, which are risk factors for falls and fractures. One of the known effects of AEDs (DPH, PB and CBZ) on the liver is to alter metabolism of vitamin D leading to a reduction in 25OHD formation. Reductions in vitamin D can result in malabsorption of calcium and a secondary hyperparathyroidism. In the present study, we demonstrated lower levels of 25OHD among patients with an incidence of 34.4% of vitamin D insufficiency. In order to improve calcium absorption when the 25-OH Vitamin D level is low, 1,25OHD is increased, a conversion step mediated by PTH. Elevation of the active metabolite of vitamin D, 1,25OHD, can stimulate bone resorption through of the receptor activated NF-KB ligand (RANKL), promoting maturation of osteoclasts26. Perhaps this mechanism can contribute to the decreased BMD observed in some patients of this study.
Besides drug-induced interference of vitamin D metabolism, geographic factors like seasons and latitudes also are important in the etiology of hypovitaminosis D. Holick27 demonstrated that in the city of Boston (latitude 44 N), there is a decrease in the skin conversion of 7-dehydrocholesterol to pre vitamin D, during the fall and almost absent in the winter months. Other studies in more sunny regions, where the dermal production of the vitamin D precursor was normal, showed neither hypovitaminosis D nor abnormalities of bone metabolism in epileptic patients9,28. For example, male AED users in Campinas (latitude 20 - 24 degrees South) showed no difference between BMD, 25OHD and 1,25OHD of patients when compared to a control group matched by age and BMI9. However, the simplistic relationship between sunny latitudes and the incidence of hypovitamosis D is not always valid. For example, in Recife, Brasil (latitude 10 -12 degrees South), Bandeira et al.29 observed that 30% of women had hypovitaminosis D. Similarly, a study performed with a group of epileptic patients living in Lebanon, showed an incidence of vitamin D deficiency close to 50%6. vitamin D deficiency is also common in Saudi Arabia30. These conflicting results indicate that other specific characteristics of patients with epilepsy can influence 25OHD levels. This was clearly the case with our patients, in whom the incidence of Vitamin D insufficiency was 34%. This was particularly noteworthy considering that subjects had their blood collected in Curitiba (latitude 24-26 degrees South) at the end of the summer and beginning of fall, a period when concentrations of 25OHD are greatest31. Several studies also have demonstrated a decrease of 25OHD serum levels in adult and pediatric patients3,4,6. As was the case in our study, others have not found a correlation between lower levels of 25OHD and BMD6. We believe that other causes of bone loss more specific to epileptic patients, independent of vitamin D levels, are involved. In our study, smoking as well as unemployment, the last associated with a reduction of non programmed physical activities, are of interest as possible other mechanisms. It is important to emphasize that the high incidence of unemployment found is a common finding in patients with chronic active epilepsy.
The normal range of serum levels of 25OHD for most assays is 10 to 20 ng/ml. However, Malabanan et al.32 suggested a normal range of 25OHD greater than 20 ng/ml justified by the fact that levels below 20 ng/ml, are typically associated with elevated levels of PTH. Another study has suggested a serum level of 25OHD of 30 ng/ml as more appropriate lower limit of normal33. The important point here is that patients can be vitamin D deficient while their 25OH D levels are still within the laboratory reference range. Such is the case with the patients reported in this study.
The finding of low levels of vitamin D in this patient population has other implications related to fracture risk, besides bone mineral density per se. Vitamin D is increasingly being implicated in other physiological processes. For example, body sway has been shown to be greater in individuals whose vitamin D levels are low. It is possible, therefore, that at the same bone density, individuals with epilepsy are at increased risk of fracture because they are at increased risk of falling. Another interesting finding of the present study was the higher incidence of low serum levels of total testosterone among male patients. No correlation was observed between this sex steroid hormone and BMD. However, as hypogonadism can lead to bone loss, this finding could be an aggravating factor.
The results of this present study call attention to the fact that patients with epilepsy who are on AEDs often demonstrate abnormalities in mineral metabolism. We were unable to identify which drug was more deleterious to bone metabolism, probably because there were a great number of patient on multiple drugs regimen and a small number of patients taking the class of no inducing AED. There were 2 points that we could related significantly to the type of AED. One was the BMD of femur neck which was lower in the group of patients that had taken PB; and second, the prevalence of DPH users among the patients with history of fractures. These two findings may be explained by the fact that these two AED have been described to present a greater number of actions on the bone metabolism. Preventive therapy should be considered for patients who are initiating treatment with AED, especially in children and teenagers, where an adequate amount of calcium and vitamin D is fundamental to the accrual of bone peak mass. Finally, a plan to treat patients who already have lost bone mass and/or who have become vitamin D deficient must be implemented. Such therapeutic maneuvers are likely to avoid fractures in the future.
In conclusion, we have shown that patients with epilepsy taking AED and who reside in Curitiba, Brazil, have decreased BMD at the lumbar spine and total femur and a substantial incidence of vitamin D insufficiency.
Acknowledgments
We would like to thank the medicine students, Carolina Ferraz da Silva and Daniel F. de Toledo for their help in getting some patients in the Outpatients Epilepsy Clinic and for reviewing their records.
Received 22 January 2004, received in final form 3 June 2004. Accepted 19 July 2004.
- 1. Engel JJ. Epileptic syndromes. In Seizures and epilepsy: Philadelphia: Davis, 1989:195-201.
- 2. Christiansen C, Rodbro P, Lund, M. Incidence of anticonvulsant osteomalacea and effect of vitamin D; controlled therapeutic trial. Br Med J 1973;4:695-701.
- 3. Hahn TJ, Hendin BA, Scharp CR, Boisseau VC, Haddad JG. Serum 25-hydroxycalciferol levels and bone mass in children on chronic anticonvulsivant therapy. N Engl J Med 1975;292:550-554.
- 4. Valimaki MJ, Tiihonen M, Laitinen K, et al, Bone mineral density measured by dual-energy X-ray absorptiometry and novel markers of bone formation and resorption in patients on antiepileptic drugs. J Bone Miner Res 1994;9:631-637.
- 5. Sato Y, Kondo I, Ishida S, et al. Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology 2001;57:445-449.
- 6. Farhat G, Yamout B, Mikati MA, et al. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology 2002;58:1348-1353.
- 7. Stephen LJ, McLellan AR, Harrison JH. Bone density and antiepileptic drugs: a case controlled study. Seizure 1999;8:339-342.
- 8. Kafali G, Erselcan T, Tanzer F. Effect of antiepileptic drugs on bone mineral density in children between ages 6 and 12 years. Clin Pediatr 1999;38:93-98.
- 9. Filardis S, Guerreiro CAM, Magna LA, Marques JF Neto. Bone mineral density, vitamin D and anticonvulsant therapy. Arq Neuropsiquiatr 2000;58:616-620.
- 10. Bouillon R, Reynaert J, Claes JH, Lissens W, De Moor P. The effect of anticonvulsant therapy on serum levels of 25-hydroxy-vitamin D, calcium, and parathyroid hormone. J Clin Endocrinol Metab 1975;41:1130-1135.
- 11. Andress DL, Ozuna J, Tirschwell D, et al. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol 2002;59:781-786.
- 12. Pack AM, Olarte LS, Morrel MM, Flasher E, Stanley RR, Shane E. Bone mineral density in an outpatient population receiving enzyme-inducing antiepileptic drugs. Epilepsy Behav 2003;4:169-174.
- 13. Verrotti A, Greco R, Latini G, Morgese G, Chiarelli F. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia 2002;43:1488-1492.
- 14. Thome JF, Cosman F, Lindsay R. Osteoporosis. In Becker KL (ed). Principles and practice of endocrinology and metabology. 2 Ed. Philadelphia: Lippincott, 1995:567-585.
- 15. Mulder JE, Kulak CAM, Shane E. Secondary osteoporosis. In Seibel MJ, Robins SP, Bilezikian JP (eds) Dynamics of bone and cartilage metabolism. New York: Academic Press 1999;527-545.
- 16. Valmadri C, Voorhees C, Litt B, Schneyer C. Practice patterns of neurologists regarding bone and mineral effects of antiepileptic drug therapy. Arch Neurol 2001;58:1369-1374.
- 17. Desai KB, Ribbans WJ, Talor GJ. Incidence of five common fracture types in an institutional epileptic population. Injury 1996;27:97-100.
- 18. Nilsson OS, Lindholm TS, Elmestedt E, Lindback A, Lindholm TC. Fracture incidence and bone disease in epileptics receiving long-term anticonvulsant drug treatment. Arch Orthop Trauma Surg 1986;105:146-149.
- 19. Vestergaard P, Tigaran S, Rejnmark L. Fracture risk is increased in epilepsy. Acta Neurol Scand 1999;99:269-275.
- 20. Lloyd T, Rollings N, Eggli DF, Kieselhorst K, Chinchilli VM Dietary caffeine intake and bone status of postmenopausal women. Am J Clin Nutr 1997;65:1826-1830.
- 21. McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly; means to defining hypovitaminosis D. Osteoporosis Int 1998;8:S3-S6.
- 22. Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137-1141.
- 23. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsy 1989;30:389-399.
- 24. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical e electroencephalographic classification of epileptic seizures. Epilepsy 1981;22:489-501.
- 25. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J 1996;312:1254-1259.
- 26. Yasuda H, Shima N, Nakagawa N. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci 1998;95:3597-3602.
- 27. Holick MF. Vitamin D: new horizons for the 21st century. Am J Clin Nutr 1994;60:619-630.
- 28. Williams C, Netzloff M, Folkerts L, Vargas A, Garnica A, Frias J. Vitamin D metabolism and anticonvulsant therapy: effect of sunshine on incidence of osteomalacia. South Med J 1984;77:834-842.
- 29. Bandeira FA, Bandeira CH, Freese EC. Occult vitamin D deficiency, and its relationship with bone mineral density, among post menopausal women in Recife, Brazil. J Bone and Miner Res 2003;18:(Suppl 2):5407.
- 30. Fonseca V, Tongia R, El-Hazmi M, Abu-Aisha H. Exposure to sunlight and vitamin D deficiency in Saudi Arabian women. Postgrad Med J 1984;60:(707):589-591.
- 31. Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab 2002;87:4952-4956.
- 32. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805-806.
- 33. Heaney RP. Sensitivity of parathyroid hormone response to calcium intake. Am J Clin Nutr. 2003;78:493.
Correspondence to
Publication Dates
-
Publication in this collection
25 Apr 2006 -
Date of issue
Dec 2004
History
-
Reviewed
03 June 2004 -
Received
22 Jan 2004 -
Accepted
19 July 2004